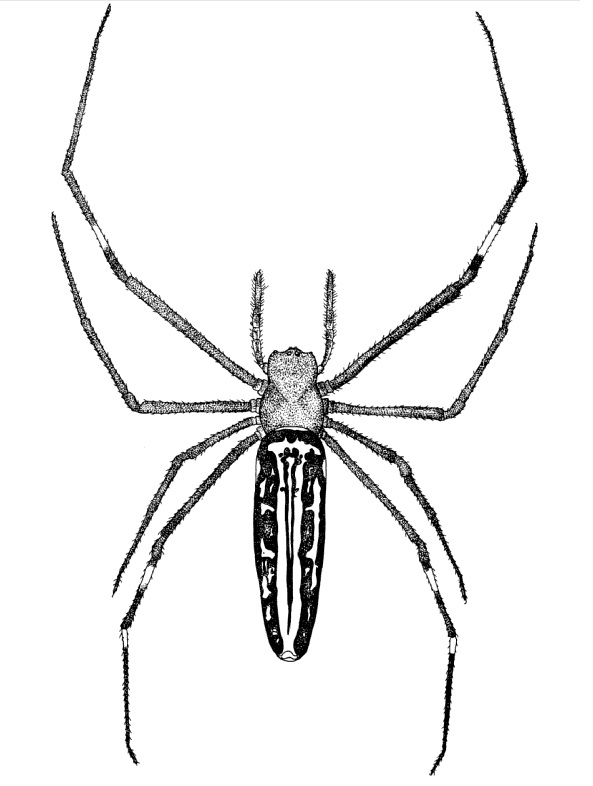
Figure 1. Female N. pilipes in Singapore (Copyright © 2008 Starmer, F.)
Table of Contents
1. Introduction
The Golden Orb Spider (Nephila pilipes) is a common species of spiders that can be found in the primary and secondary forests of Singapore.[1] It is probably best known to the public for its ability to build large, spectacular webs with spider silk that is stronger than kevlar. However, although large webs might mean more prey caught, the trade-off would be its susceptibility to parasites (See Section 3.3). Like all spiders from the family Nephilidae, female gigantism (See Section 3.5) is extremely pronounced[2] and this results in the large sizes of mature female spiders, achieving body lengths of 40mm to 50mm.[3] Despite its huge size and common status, it is surprising to note that there are not many studies which focuses on its biology, unlike its American counterpart (Nephila clavipes). Hence, this species page hopes to serve two purposes: (1) as a interesting read for whoever that is interested in finding out more about its biology and (2) for spider enthusiasts who are keen to learn about its anatomy and how to go about identifying one.
Author's notes to readersThis page is almost always referring to the female N. pilipes. References to males will be explicitly stated.A glossary of terms would be useful in the understanding of terms used in this page.This page is optimised for view at 1920x1080 resolution. Viewing at smaller resolutions may result in warped alignments and missing content. Zooming out (Ctrl + -) might help with alignment issues.If you would like to contact me regarding inaccuracies/copyrights/dead links etc., you can do so here.
2. Distribution
2.1 Global Distribution
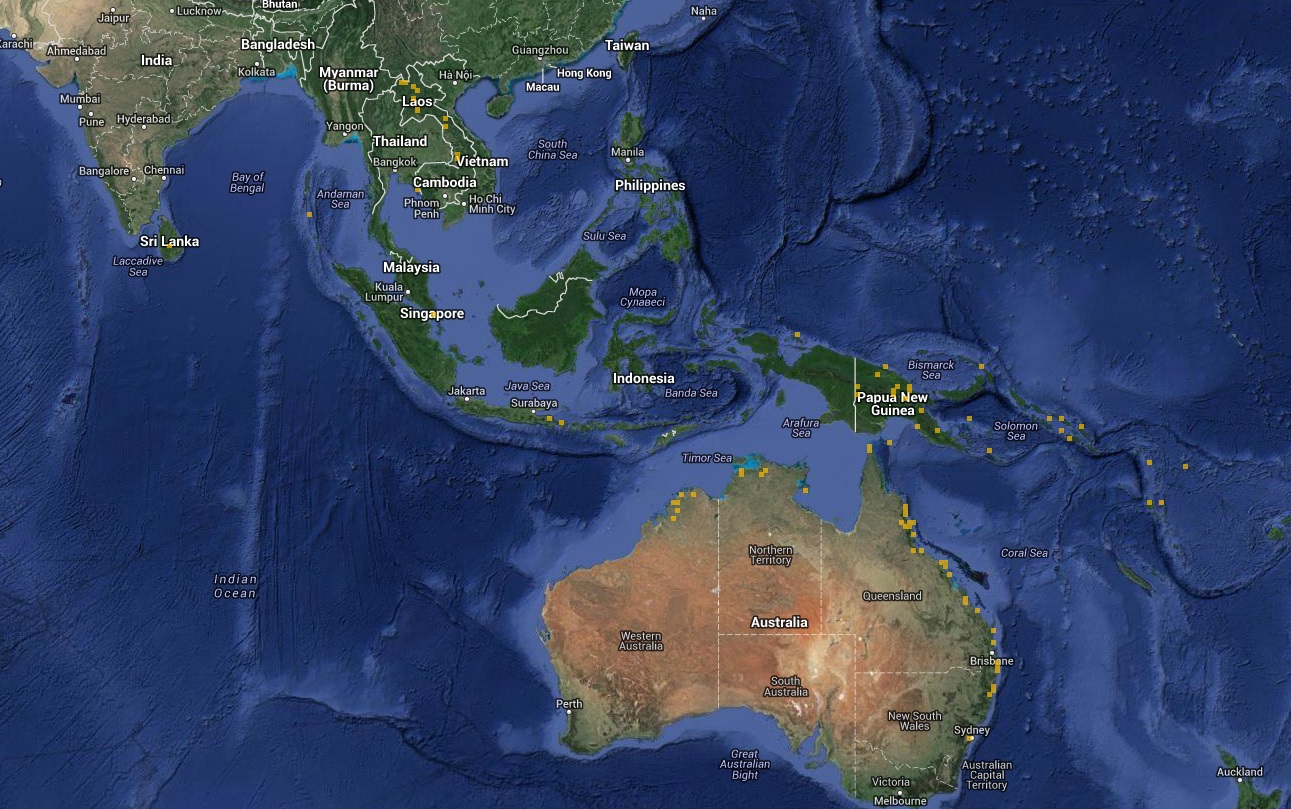 |
| Figure 2. Global distribution of N. pilipes indicated by the yellow dots (Picture from Encyclopedia of Life) |
The global distribution of N. pilipes, based on occurrence reports by the Global Biodiversity Information Facility (GBIF) network, includes Singapore, Sri Lanka, Thailand, Laos, Indonesia, Papua New Guinea, Northern and Eastern Australia. However, this distribution is not exhaustive as N. pilipes is also known to be present in Myanmar, India, China, Japan, the Philippines and South Africa.[4][5][6]
2.2 Local Distribution
View List of known locations of N. pilipes in Singapore in a larger map
N. pilipes is considered a common species of golden orb spider in Singapore. Hence, they can be found in the various locations indicated in the interactive google map above:
- Bukit Timah Nature Reserve
- Sungei Buloh Wetland Reserve
- Central Catchment Nature Reserve
- MacRitchie Reservoir Park
- Bedok Reservoir Park
- Seletar Reservoir Park
- Bukit Batok Nature Park
- Dairy Farm Nature Park
- Kent Ridge Park
- Hort Park
- Pasir Ris Town Park
- Singapore Botanical Gardens
- Pulau Ubin
3. Biology
3.1 General Orb-Web Construction
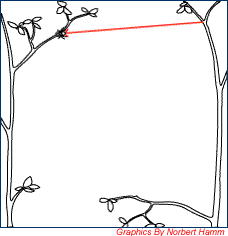 |
In order to start building the orb web, the bridge line is first built. This is considered the hardest step in the web construction process since it requires some luck. N. pilipes employs the kiting method to build the bridge line and this is done by releasing a thin and sticky silk thread from its spinnerets. Since the thread is lightweight, it is easily carried by wind until it attaches onto something suitable. In the illustration to the left, the silk thread is attached onto an opposite branch thus forming a proper bridge line. The spider then reinforces the bridge line by walking back and forth along the bridge line whilst spinning more threads. The bridge line has to be strong in order to withstand the weight of the entire web.[7] |
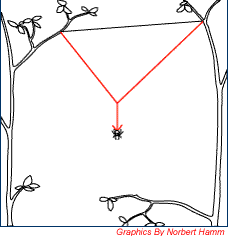 |
The spider then sets the third anchor point by hanging down from the middle of the bridge line hence forming a "Y" frame. The "Y" frame forms the first three radial lines of the web. |
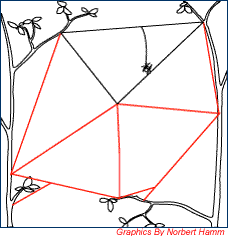 Figure 5. Step 3 (Silk Framework) |
A silk framework is then created to provide an anchor for more radial lines. The silk used to make the supportive frame and radials are not sticky. |
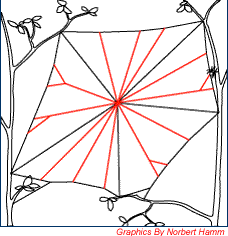 Figure 6. Step 4 (Radial Lines) |
More radial lines are added to complete the supportive structure of the web. The radial lines start from the middle to the outer frame of the web and they cannot be placed too far apart, so that the spider can still cross them. This is essential since the spider walks on the supportive structure so as not to get entangled in the sticky silk.[8] |
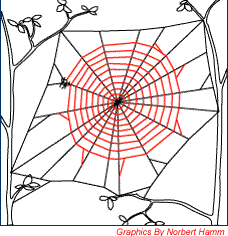 Figure 7. Step 5 (Dry Silk Spiral) |
Starting from the middle of the web, a non-sticky silk spiral is spun towards the frame of the web. This serves to hold the radial lines apart and also as a reference for the spider to spin the sticky spiral. |
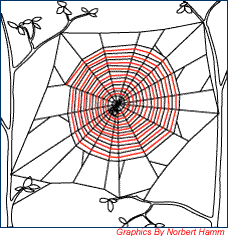 Figure 8. Step 6 (Sticky Spiral) |
The sticky spiral is spun in reverse direction and the spider ends in the middle of the web where it waits for prey to get entangled in its web.[9] |
| Figures 3 - 8. Construction of a basic orb web(Copyright © 2002 Hamm, N.) |
3.1.1 Orb-Web of N. pilipes
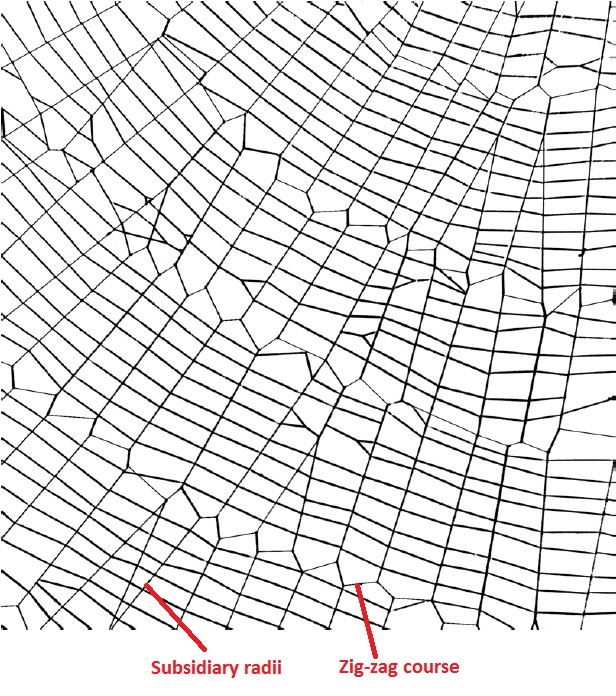 |
Over time, web construction and design have been modified resulting in many variations between species. Hence, the orb web of N. pilipes differs from the typical orb-web in a few ways:
|
| Figure 9. Web below the hub showing the zig-zag course and subsidiary radii (Zschokke, 2000) |
YouTube Video 1: Grasshopper warbler caught in the web of N. pilipes in Tungareshwar Wildlife Sanctuary, Mumbai, India. (Copyright © 2007 sanal nair)
3.2 Feeding Behaviour
The feeding behaviour of N. pilipes has been quite well studied by Robinson & Robinson (1973) and their findings are very briefly summarised here.
- Predatory Position at the Hub: Prior to the prey hitting the web, the spider adopts a position at the center of the hub with all the leg pairs separate from each other, in a bilaterally symmetrical position. The tarsi are all in contact with the web so that the spider can be alerted in the event whereby a prey hits the web. After an object hits the web, the spider re-orientates itself in the general direction of the object and may either pluck at the web or approach the object directly. The plucking of the web behaviour has been theorised to be the spider's way of telling if the object is living or dead[11] or to pinpoint the exact location of the object on the web.[12] Approach plucking may occur whereby the spider plucks its web as it approaches the object. This process may be abandoned if the object starts moving in response.
- Approaches to the prey: The approaches vary a lot depending on the situation. Generally, if the object is actively moving, the spider would make a rapid approach, with the exception of a large prey item. The spider may also start slow first and accelerate when the object starts moving. Before the approach, the spider would attach a dragline silk to the hub which facilitates its return to the hub.
- Contact with prey: Upon contact with the prey, the spider touches and taps the prey with Legs I and may back up or even retreat if the prey is large. In most cases, the touching is followed by the attack stance over the prey. This touching behaviour has been thought to be due to the spider's need to determine the exact location to bite its prey.
- Attack Behaviour: There are three types of attack behaviours.
- Long Bite: The predominant mode of attack would be the long (sustained) bite whereby the spider inserts its fangs into the prey for an extended period. There are cases where the spider has had to re-position its bite due to the first being ineffectual. This happens mostly in active or heavily sclerotised prey.
- Bite and Back-off: In this mode of attack, the spider lunges and bites for a short period of less than 5 seconds (usually significantly shorter) before releasing and backing off. Several repetitions may occur before the final (long) bite is delivered. This method is usually observed when prey size is large.
- Seize and Pull-out: This mode of attack tends to be used when prey is small and light. The prey seized in the jaws of the spider and pulled out of the web. However, it is not clear if venom is used when employing this mode of attack.
- Removal of Prey from Capture Site: The prey can either be pulled out of the web and transported to the hub in the jaws before being wrapped in silk or wrapped in silk at the point of capture (very large prey or prey that cannot be freed by pulling).
- Transportation to the Hub: When the prey is small and light, the preferred choice of transport back to the hub is to ascend backwards with the prey in jaws, via the dragline silk. It is still unclear why majority of observed spiders do this despite turning around and walking/running back to the hub being significantly faster than the former method. If the prey is large, it is usually transported via a silk thread suspended from the spinnerets. Because of the sloping gradient of the web, the prey would hang away and not get tangled in the web.
- Prey at the Hub: Prey that were brought back to the hub in the spider's jaws will be wrapped in silk. Prey transported via a silk thread will be attached to the hub via the original silk thread (See Video 2).
- Feeding Method: The prey caught by the spiders are fed upon by straightforward suctorial feeding, evidence from the remains of prey items.[3]
YouTube Video 2: Video showing the opportunistic mating by the male while the female is feeding on a large prey. (Copyright © 2011 Marek Bialoglowy)
3.3 Kleptoparasitism
| Kleptoparasitism is the stealing of food from a host who has spent energy to capture the prey. It is a foraging behavior that is widespread in the animal kingdom.[13] Spiders from the genus Nephila are especially susceptible to kleptoparasites (usually from genus Argyrodes) for several reasons:
The dependence of Argyrodes on Nephila have been well documented in Singapore[16], America[17] and Australia[18]. Their relationship has been shown to be kleptoparasitic (negative effect to the fitness of the host) instead of commensal (no negative effect to the fitness of the host) as previously suggested[19]. The study in Singapore showed that A. flavescens had a detrimental effect on host N. pilipes, reducing host weight gain, increasing web damage, rate of web relocation and ultimately mortality.[16] |
|
| Figure 10. Many juvenile A. flavescens on the web of N. pilipes (Copyright © 2008 Starmer, F.) |
3.4 Reproduction
| Finding the female: The exact process by which the male locates the female is still not well studied but it has been proposed that short distance pheromonal attraction might be present. Another possibility is that there is some kind of chemical mediated signals which females leave on the silk of their webs that are species specific and recognised by the males.[3][5] Courtship: The time taken from the initial approach to the actual process of copulation may range from as short as a minute to as long as a few days. Courtships that happen fast are usually due to the males being opportunistic and mate when the female is in its most vulnerable state, which is when they are feeding or in the process of moulting.[3] In all other cases of courtship, the process can be broken down into distinct phases.
Movement towards the epigyne and copulation: This stage may happen intermittently during silk deposition. The male inserts an embolus of his pedipalp into the female genital, one at a time. This may last from a few seconds to over a minute, although the former might be representative of a failed attempt with no sperm transfer. Both the pedipalps are used several times alternatively, as with most spiders.[21] |
|
| Figure 11. Ventral view of the female N. pilipes showing copulation by the male (Copyright 2013 © Rentz, D.) |
YouTube Video 3: Video showing the silk deposition of the male on the female's dorsum (Copyright © 2011 Smithsonian Science)
3.5 Sexual Size Dimorphism (SSD)
There has been some debate about whether SSD is caused by male dwarfism or female gigantism in N. pilipes[22] but newer studies seem to lend weight to the latter.[56]
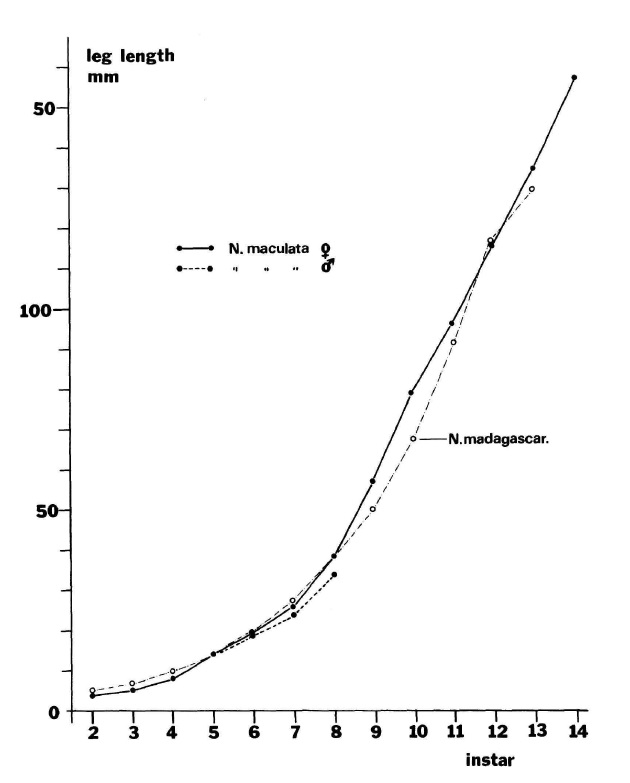 |
In order to understand SSD in females, there is a need to have a basic understanding of the life cycle of both the male and female N. pilipes and its different moulting stages. N. pilipes like most spiders, moult whenever their body grows too large for its exoskeleton. Hence, new spiderlings start at instar 1 (Stage 1) and moult into instars 2 (Stage 2). The graph in Figure 12 features an external study on N. madagascariensis as a comparison and to reaffirm the accuracy of the growth of N. pilipes despite having slightly differing methodologies.[23] Males: From Figure 12, males start at instar 1 and moult 7 times, increasing in size until instar 8 where it reaches sexual maturity and never moult again. Upon reaching sexual maturity, males lose their ability to spin sticky silk and hence, must resort to kleptoparasitism to survive.[23] The mature males in the Nephila genus have been found to always be more or less the same size[24]suggesting that this optimal size is an ancestral trait. Moreover, being small is advantageous since they have a need to turn to kleptoparasitism. Females: From Figure 12, females start at instar 1 and moult 13 times, increasing in size until instar 14 where it reaches sexual maturity and never moult again. However, the authors note that there is a possibility that some may reach maturity at the 12th or 13th instar, likely to be dependent on the rate of prey capture.[23] The mature females can have variations in sizes. There are of course advantages and disadvantages for females to have a delayed maturation (mature at a later instar). Advantages: They outgrow predators well before maturation and the increase in size also translates to increased fecundity. Disadvantages: Increased development time and risk of mortality due to seasonal shifts. Seasonal shifts are only applicable when the spider is in an environment where seasons cause significant temperature changes. In such a scenario, a delayed maturation might have severe consequences as the spider might die off without maturing and producing offspring before the season changes. It has been argued that given the relatively long development time (6 months) of N. pilipes and the significant benefit from the increased number of eggs the female can lay, the additional 2 weeks of development time can be overlooked. This supports the fecundity selection hypothesis put forward by Darwin in 1871 and is probably why female gigantism is the more likely driver of female biased SSD in N. pilipes.[22] Having said that, the mechanics of female biased SSD is still poorly understood and more work needs to be done. A very recent study by Kuntner et al. (2012) found that the females actually do undergo post-maturity moulting without shedding their genitals. They attribute this finding to the following reasons (1) a response to shorter copulation times (less sperm accumulated) or (2) as a chemical signal to attract more males.[56] |
| Figure 12. Graph showing the leg length (mm) vs instar stage of male and female N. pilipes (Robinson & Robinson, 1976) |
4. Human Applications (Biomimicry)
Biomimicry or biomimetics is essentially to recognise good design from nature, then understand and try to adapt it to solve complex problems that we face in today's world. Since the stone age, men have looked to nature for answers to problems. Animal fur inspired the invention of winter coats, flight of birds inspired the invention of planes by the Wright Brothers[25] and even at present, self cooling termite mounds inspired the energy efficient Eastgate Center in Zimbabwe.[26]Leonardo da Vinci also recognised the beauty of nature as he wrote "Though human ingenuity may make various inventions which, by the help of various machines answering the same end, it will never devise any inventions more beautiful, nor more simple, nor more to the purpose than Nature does".[27]
Useful Properties of Spider Silk
Scientists have tried to study and learn more about spider silk because it has many desirable qualities of a polymer fibre:
- Strength: Spider silk is approximately 5 times stronger than steel per unit weight, owing to its low density.[28]
- Toughness: Toughness is the combination of strength and ductility. Spider silk is well known for its toughness and is even stronger than kevlar, which are considered benchmarks of modern polymer fibre technology.[29]
- Temperature resistant: Dragline silks are able to retain their properties at temperatures ranging from -40°C to 220°C.[30]
- Torsional properties: Torsional properties of the spider silk refers to the twisting of the silk. Spider silk has unrivalled torsional qualities which is why the spider does not twist when suspended from its silk thread.[31]
Possible Applications
With these amazing properties, the medical industry may benefit from stronger sutures, durable artificial ligaments and even neuronal regeneration procedures.[32]The military industry may benefit from lighter and faster vehicles, planes and boats, lighter bulletproof vests. The engineering industry may benefit from more durable constructs like bridge cables, engines etc. Theoretically, the possibilities are only limited by man's creativity.
Obstacles
Having said that, the current major obstacle in the field of study of spider silk is that no one has found an efficient method of mass producing spider silk. The current approaches are:
- Genetically alter silkworms to produce spider silk instead since silkworms are easier to handle in captivity and produce larger quantities of silk compared to spiders.[33][34]
- Genetically alter goats to produce spider silk protein in their milk which is then used to spin spider silk.[34][35]
- Genetically alter the bacteria, Escherichia coli with the genes of spider silk in order to make spider silk directly.[36]
- Genetically alter alfalfa plants to produce spider silk since they have a high protein content and are widely distributed.[34][35]
5. Description
Although there have been observations of variations of N. pilipes, they are found to differ only in terms of colouration, depending on locality, but not morphology.[5] This section describes the morphology of the spider with respect to the local variation of N. pilipes. Due to the lack of studies on N. pilipes with respect to N. clavipes, some of the illustrations are of the latter but they essentially fulfill the purpose of the description and explanation. A glossary of terms would be useful in understanding the terms used in this section.
5.1 Morphology of N. pilipes (Female)
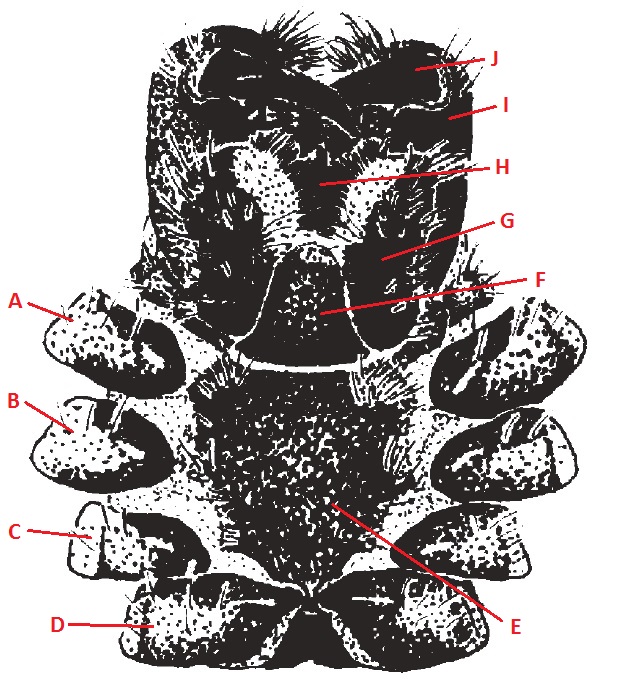 |
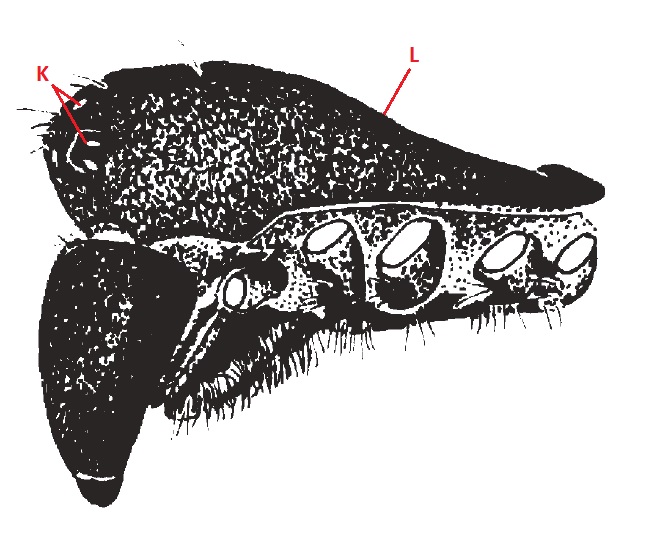 |
| Figure 13. Ventral view of cephalothorax of N. pilipes (Harvey et al., 2007) |
Figure 14. Lateral view of cephalothorax of N. pilipes (Harvey et al., 2007) |
Table 1. Description of the general morphology of N. pilipes with labels based on Figures 3 and 4.[5][6][37][38]
| Label |
Name |
Description |
| A |
Coxa I |
First segment of the first pair of legs, counting from the anterior end. |
| B |
Coxa II |
First segment of the second pair of legs, counting from the anterior end. |
| C |
Coxa III |
First segment of the third pair of legs, counting from the anterior end. |
| D |
Coxa IV |
First segment of the fourth pair of legs, counting from the anterior end. See Section 5.1.1 |
| E |
Sternum |
Black; cordate, extending between coxae IV; smooth, except for small, glabrous protuberances adjacent to coxae I, II and III. |
| F |
Labium |
Black; much longer than broad, anteriorly rounded. |
| G |
Maxilla |
Dark brown. Part of the spider mouthparts. Involved in manipulation and ingestion of prey. |
| H |
Rostrum |
Part of the spider mouthparts. Involved in manipulation and ingestion of prey. Covered with hairs to filter food while feeding. |
| I |
Chelicera |
Part of the spider mouthparts. Very dark brown; cheliceral boss covered with tuberculations; fang furrow with three large teeth on anterior furrow (central tooth largest), four large teeth on posterior furrow; cheliceral denticles present between tooth rows. |
| J |
Fang |
Joint to the chelicera and aids the spider in piercing the prey to inject the venom. |
| K |
Eyes |
Eight eyes arranged in two recurved rows. See Section 5.1.2 |
| L |
Carapace |
The exoskeleton in the dorsal portion covering the cephalothorax. Very dark brown, nearly black, postero-lateral corners with indistinct yellow markings; evenly covered with small, pale setae; fovea a broad depressionl; dorso-medial horns represented by extremely small roundere protuberances; antero-lateral margins with red-orange crenulated mound that opposes cheliceral boss; chilum present, medially divided. |
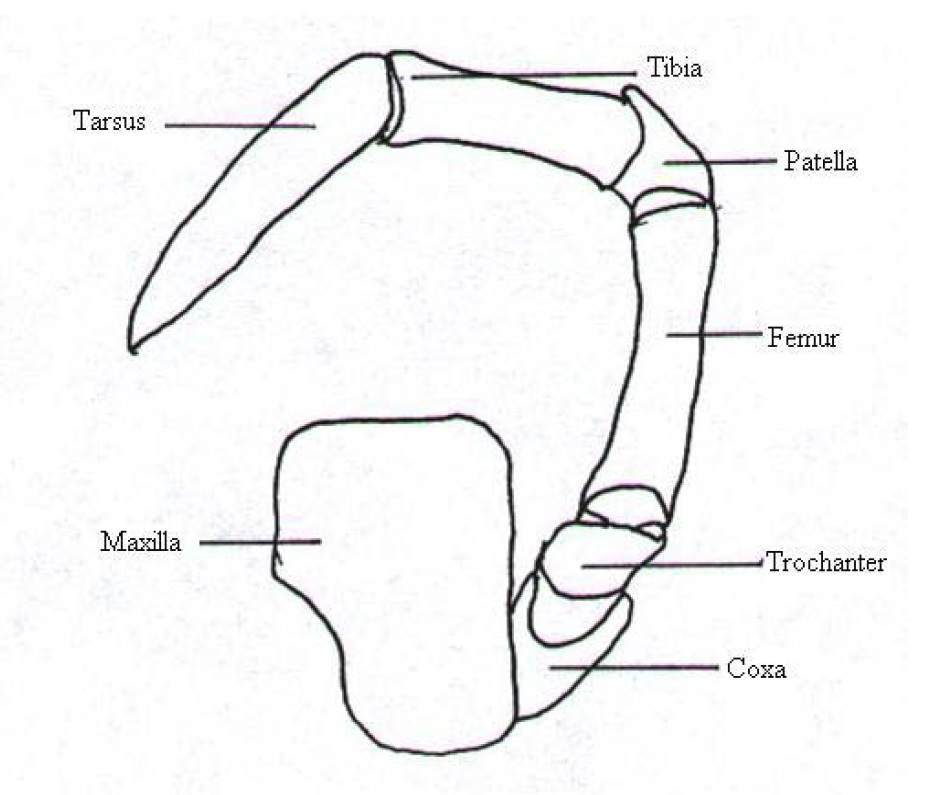
5.1.1 Legs
 |
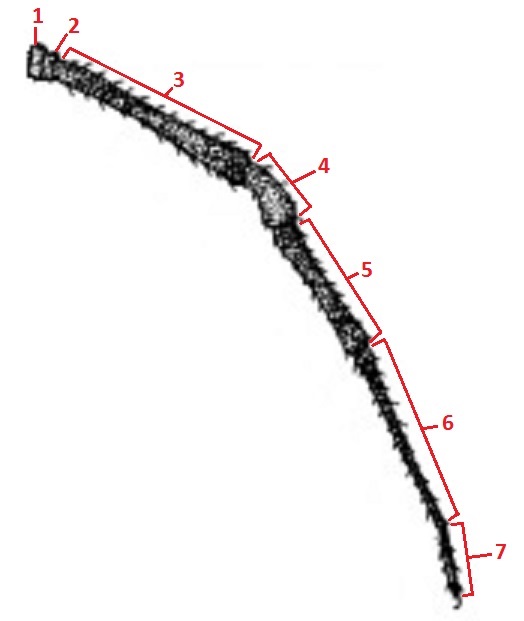 |
| Figure 15. Dorsal view of female N. pilipes (Barrion and Litsinger, 1995) |
Figure 16. Magnified view of leg III from Figure 15 (Barrion and Litsinger, 1995) |
N. pilipes, like any other spider has four pairs of legs that are named I - IV starting from the anterior end. Usually, leg I are the longest legs out of the four pairs of legs. This is followed by leg IV, which are longer than leg II but the difference is very slight. Leg III are the shortest legs and have been observed to be approximately half the length of that of legs I. Despite the frequent occurrences of asymmetry in leg lengths, the order still holds true in most cases.[3] The legs can be broken down into seven segments (Fig. 16) and are named as such (Table 2). The coxae are generally black in colour. All other lateral margins are dark brown and centrally yellow-brown. All other segments range from very dark brown to black. The inter-segmental membranes are bright yellow.[5]
Table 2. Description of the leg morphology of N. pilipes with labels based on Figure 16.[5][6][37]
| Label |
Name |
Description |
| 1 |
Coxa |
First leg segment. Between body and trochanter. |
| 2 |
Trochanter |
Second leg segment. Between coxa and femur. |
| 3 |
Femur |
Third leg segment. Between trochanter and patella. |
| 4 |
Patella |
Fourth leg segment. Between femur and tibia. |
| 5 |
Tibia |
Fifth leg segment. Between patella and metatarsus. |
| 6 |
Metatarsus |
Sixth leg segment. Between tibia and tarsus. |
| 7 |
Tarsus |
Seventh leg segment. Between metatarsus and tarsal claws. |
5.1.2 Cephalothorax
Eyes
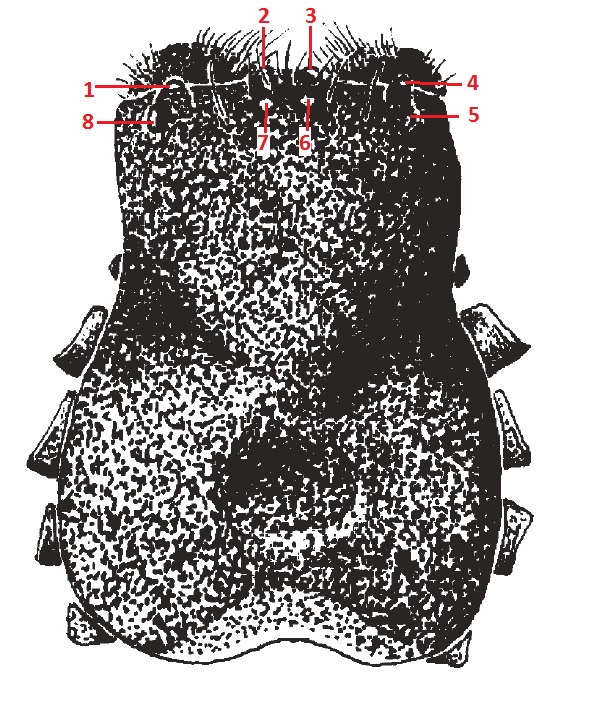 |
 |
| Figure 17. Dorsal view of cephalothorax of N. pilipes (Harvey et al., 2007) |
Figure 18. Anterior view of cephalothorax of N. pilipes (Copyright © 2013 Hewett, N.) |
The colour, number and arrangement of the eyes of a spider are useful features to aid identification.[37] The eight eyes of N. pilipes are arranged in two rows of four (Fig. 17, 18). They are commonly referred to in groups and their respective abbreviations as shown in Table 3.
Table 3. List of grouping names and abbreviations of the eyes of N. pilipes.[6][37]
| Group Name |
Eye Numbers |
| Anterior Lateral Eyes (ALE) |
1, 4 |
| Anterior Median Eyes (AME) |
2, 3 |
| Posterior Lateral Eyes (PLE) |
5, 8 |
| Posterior Median Eyes (PME) |
6, 7 |
Chelicerae
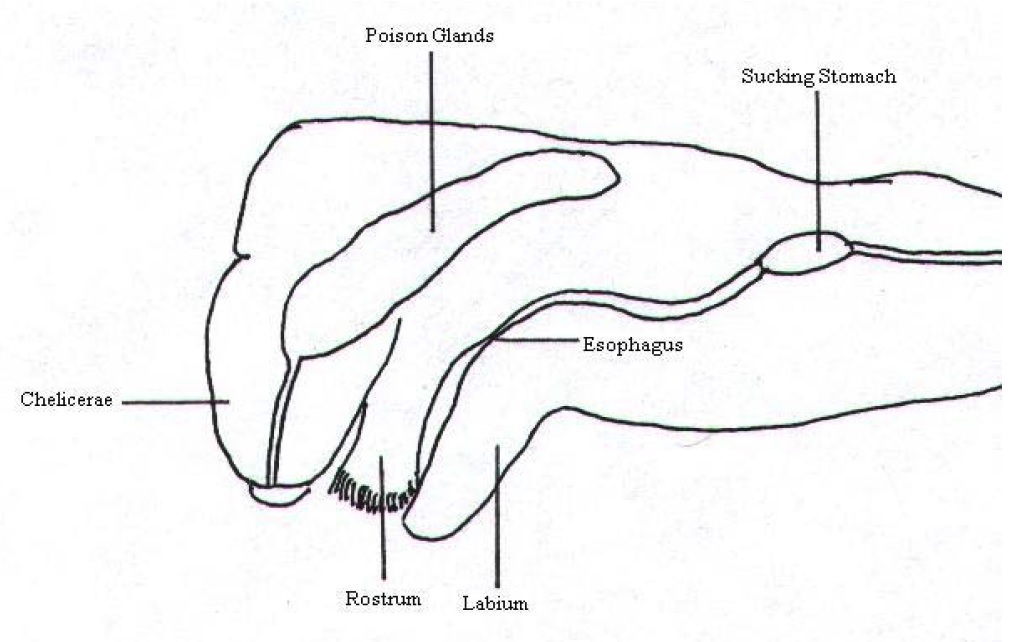 |
Chelicerae (Fig. 18, 19) are the first pair of appendages of the cephalothorax. They are made up of a basal region, which is joint to the cephalothorax and a fang (Fig. 13, 18) which is joint to the basal region.[6] Poison Glands The poison glands which produce the neurotoxin where N. pilipes utilises to immobilise its prey is found in the two segments of the chelicerae. The glands in the basal region are surrounded by muscles and thus, this allows the spider to administer different amounts of venom depending on the situation and prey type.[6] The venom is injected into the prey via openings in the tip of the fangs.[37] Cuticular Teeth Cuticular teeth may be found on the chelicerae of some species of spiders. Hence, the absence or presence and the number of teeth can help in identification. In addition, the differences in direction of fang opening is another character that can aid in identification.[37] |
| Figure 19. Lateral cross section of the cephalothorax of N. clavipes (Starmer, n.d.) |
Pedipalps
 |
Pedipalps are the second pair of appendages of the cephalothorax, which are anterior to the legs and just after the chelicerae.[37] It has the same segmentation as the legs (Section 5.1.1) except for the metatarsus which is lost. The trochanter, femur, patella and tibia can be yellowish-brown (Fig. 1, 10) or red (Fig. 18). The tarsus is dark brown to black in colour. |
| Figure 20. Illustration of pedipalp of female N. clavipes (Starmer, n.d.) |
5.1.3 Opisthosoma
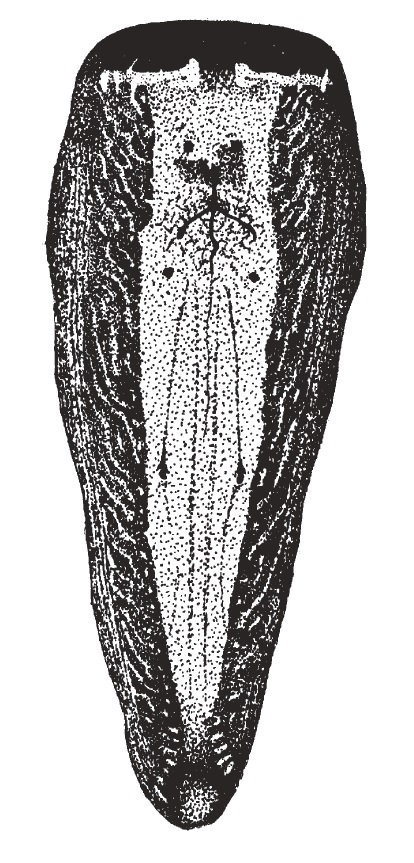 |
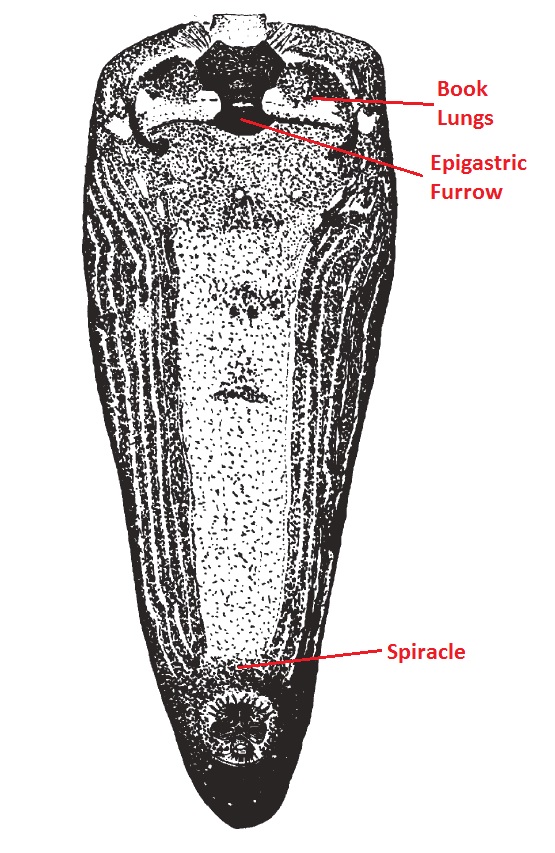 |
The opisthosoma is one of the two body segments which is nearer to the posterior end. It consists of nine abdominal segments that are fused and thus, not easily distinguishable. The pedicle is the first segment and the anal tubercle last. The exoskeleton of the opisthosoma is very thin and usually not heavily sclerotized. The opisthosoma houses the spider’s breathing apparatuses, reproductive organs and silk producing organs.[5] Colourations The dorsum is mostly dark yellow-brown, with conspicuous yellow stripes anteriorly (Fig. 1, 10). The stripes are much longer than they are broad.[5] However, there seems to be a great variation in the yellow colouration of the opisthosoma. Distinct yellow stripes were observed in N. pilipes from North-Western Australia, most of Asia, New Guinea and Solomon Islands. Those observed without stripes or with indistinct markings occurred in eastern Australia and parts of montane New Guinea.[3] There were also observations of spots instead of stripes in New Britain, New Ireland, Solomon Islands and Samoa.[5] Respiratory Organs Spiders have two types of respiratory organs, which are located on the venter of the opisthosoma. They are a pair of book lungs (Fig. 11) and a spiracle, which is located towards the anterior and posterior of the venter of the opisthosoma respectively. The presence of book lungs and the placement of the spiracle on the opisthosoma are useful in the identification and classification of spiders.[5] Sexual Organ (Epigyne) The epigyne is the external genital structure of females. In N. pilipes, it is very wide and narrow, with paired lateral indentations and a deep depression across the posterior margin. It is associated with two ovoid spermathecae and short copulatory and insemination ducts.[5] It can be reached through the epigastric furrow and serves to direct the male palpal organ to deposit their sperm in spermathecae pouches. Since the epigyne differs a lot, even within closely related species, it is useful in identification and classification.[39] |
| Figure 21. Dorsal view of opisthosoma of female N. pilipes (Harvey et al., 2007) |
Figure 22. Ventral view of opisthosoma of female N. pilipes (Harvey et al., 2007) |
Spinnerets
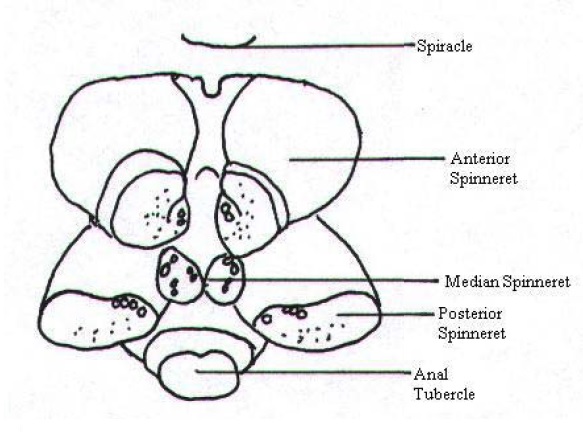 |
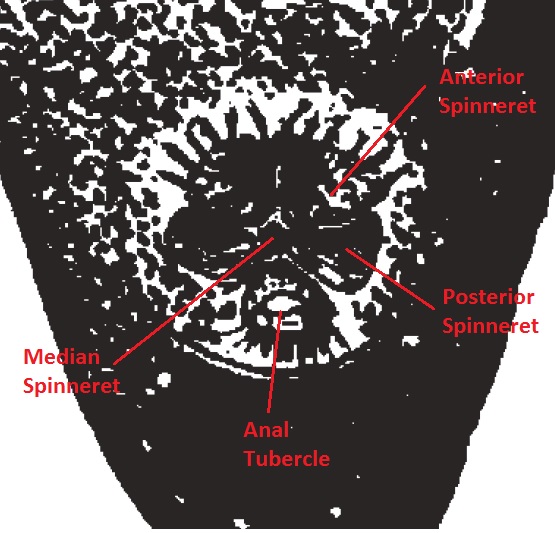 |
Spinnerets are organs that are located at the posterior end on the venter of the opisthosoma. Like most spiders, N. pilipes has a total of six spinnerets (some spiders have two, four or eight) which corresponds to six types of silk glands, each performing a different function. Each spinneret is supplied by a minimum of two silk glands and are capable of working in an independent yet concerted manner to spin spider silk.[37] Arrangement Spinnerets are usually arranged in a circular pattern and grouped into anterior, median and posterior spinnerets (Fig. 23, 24). Spinnerets vary in length, shape, and arrangement. Three other structures of note are found near the spinnerets.[37] Anal Tubercle The anal tubercle is a small tubercle carrying the anal opening. It is easy to mistake the anal tubercle as the seventh spinneret since it is found in very close proximity to the posterior spinnerets.[37] |
| Figure 23. Ventral view of spinneret of female N. clavipes (Starmer, n.d.) |
Figure 24. Enlarged view of spinneret from Figure 22 (Harvey et al., 2007) |
5.2 Morphology of N. pilipes (Male)
Studies on N. pilipes have largely been concentrated on the females and this is not surprising since the females are much larger and they spin large orb-webs. Moreover, they tend to be more colourful than their male counterparts. The task of distinguishing the males of N. pilipes from the females is clear and straightforward. Nevertheless, there is still a need to know the morphology of the male N. pilipes due to the susceptibility of Nephila to kleptoparasitism (Section 3.3). Argyrodes (usually A. flavescens)[16] are at first glace pretty similar to the male N. pilipes and closer examination is required in order to tell them apart.
| Figure 25. Anterior view of male N. pilipes from Mandalay, Queensland, Australia (Copyright © 2013 Pearson & Pearson) 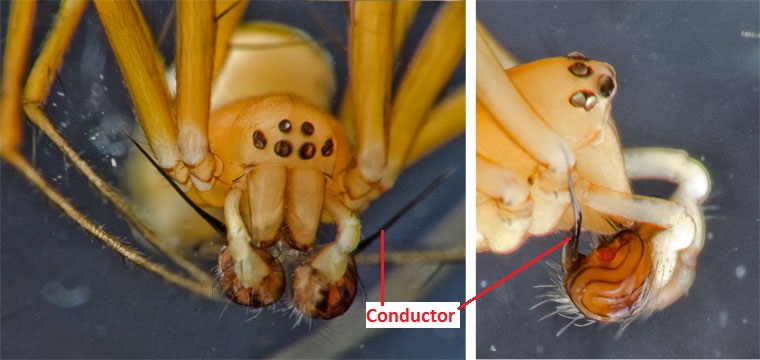 |
Comparison between both sexes The males and females of N. pilipes are largely similar in terms of morphology. However, there are a few differences between the two sexes. Size The males of N. pilipes are only approximately a tenth as big as the females. The males grow up to a body size length of 5 - 6mm while the females can grow up to body size lengths of 40 - 50mm.[1][3][5] Colour The males are usually dull looking in a translucent reddish-orange coloration, often with black coloured tarsi (Fig. 25)[3] while the females usually have yellow coloured striations on the dorsum of the opisthosoma (Fig. 1, 29). Pedipalps The tarsus of the pedipalps of the males have been modified into sex organs (palpal organs) used for sperm transfer. Sperm is produced in testes and then transferred to the palpal structure in which the males uses to mate with the female. The modification of the tarsus can differ considerably between species and hence, is a useful feature for identification and classification.[6][8] Conductor The conductor (Fig. 26) is a sclerotized hook of the pedipalp which serves to anchor the male to the female during copulation.[10] It is a character which is present only in the male and hence, is useful for identification. Spination Leg spination (Fig. 25) is a clear feature observed only in the males of N. pilipes. The function of the spines are unknown. |
Comparison between males of N. pilipes (Fig. 25) and A. flavescens (Fig. 27) There are 3 features one can rely on in order to distinguish between the two species: Pedipalps: The pedipalps of the male N. pilipes are a lot bigger than that of A. flavescens. Spination: Spination occurs only on the legs of the male N. pilipes but not that of A. flavescens. Opisthosoma: The opisthosoma of the male N. pilipes is more cylindrical instead of round, as that of A. flavescens. In addition, the silver colourations seen on the opisthosoma of A. flavescens is characteristic of the species.  |
| Figure 26. Anterior (Left) and lateral (Right) view of male N. pilipes preserved in alcohol (Copyright 2013 © Whyte, R.) |
Figure 27. Dorsal view of A. flavescens for comparison (Copyright © 2008 Starmer, F.) |
6. Diagnosis
| Figure 28. Ventral view of female N. pilipes (Copyright © 2010 Benjamint444) |
Figure 29. Dorsal view of female N. pilipes (Copyright © 2010 Benjamint444) |
Figure 30. Dorsal view of female N. antipodiana (Copyright © 2008 Starmer, F.) |
N. pilipes and N. antipodiana are the two more common species from the Nephila genus that can be found in Singapore, hence the need to know how to differentiate the two species. At first glance, they may be mistaken to be same species since both spin a golden orb-web and exhibit female gigantism. However, they can be clearly differentiated in a few ways:
- A characteristic feature of N. pilipes is the yellow spot colouration on the underside of its legs (Fig 10, 28) whereas N. antipodiana has completely black/reddish-brown legs.[40]
- The abdomen of N. pilipes in Singapore is yellowish-black in colour. From dorsal view, the median part is yellow with with a black longitudinal band through the middle.[6] The abdomen of N. antipodiana is usually with yellow spots instead (Fig. 30).
- The pedipalps of N. pilipes is sometimes red (Fig. 8) or yellowish-brown (Fig 1, 10) in colour whereas N. antipodiana has completely black pedipalps.[40]
- There are distinct thoracic tubercles on the female of N. antipodiana but not that of N. pilipes.
- The leg formula (longest to shortest) for N. pilipes is 1423 but that of N. antipodiana is 1243.[6]
- The abdomen of N. antipodiana (Fig. 30) is usually wider and shorter than that of N. pilipes (Fig. 29).
7. Taxonomy
7.1 Phylogenetics
The taxonomic history of the genus Nephila has seen it being shifted a few times before recent studies put them in their present family Nephilidae. In the past, Nephila was regarded as part of the family Araneidae which consists of spiders which constructed orb-webs. Subsequently, Nephila was included in the family Tetragnathidae in 1989[42] which was backed up by later studies in 1995, using newer data from spinneret silk gland spigots, morphological and behavioural characteristics.[43] Then in 2004, a phylogenetic study based on 12S and 18S rRNA and 3'-end partial cDNA of major ampullate spidroin-1 (MaSp1) gene sequences resulted in Nephila being put back under the family Araneidae[44] before a more recent detailed study of 197 morphological and 34 behavioural characters in 2008[45] concluded with Nephila being placed back into the family Nephilidae.[5] This placement is again confirmed in 2011.[46]
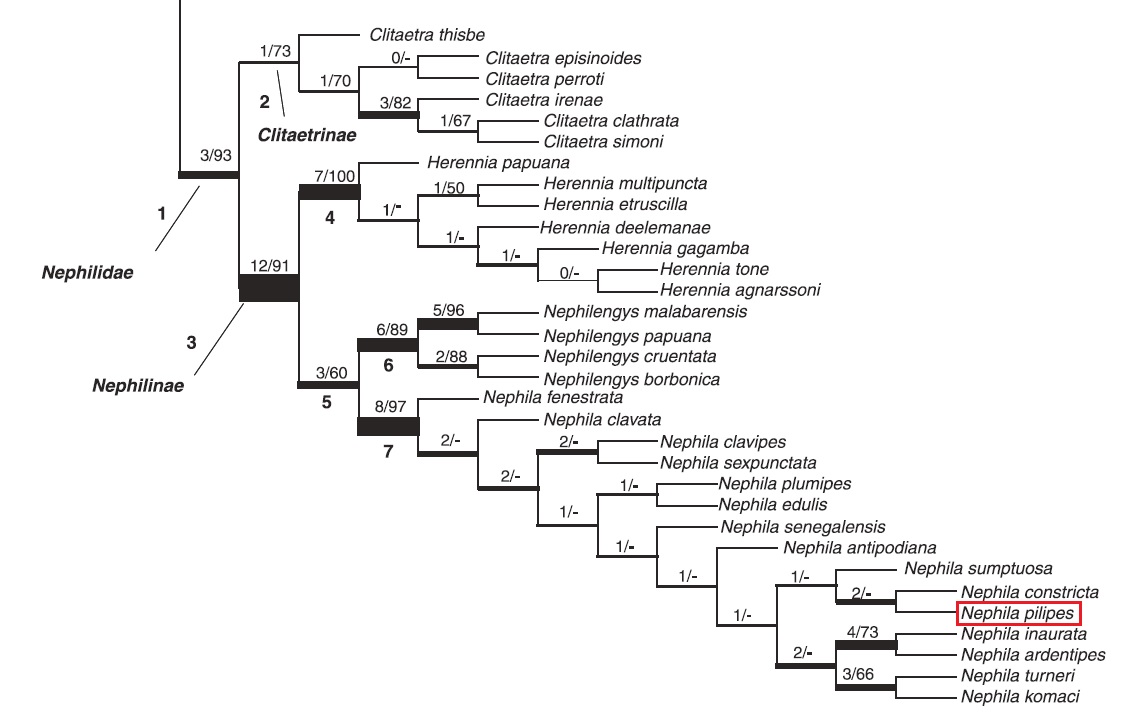
Figure 31. Parsimony tree showing the position of N. pilipes in the family Nephilidae and subfamily Nephilinae (Kuntner et al., 2008)
7.2 Names
Taxonavigation
This taxonavigation is based on the recent study by Kuntner et al. (2008).[45]
| Kingdom |
Animalia |
| Phylum |
Arthropoda |
| Class |
Arachnida |
| Order |
Araneae |
| Superfamily |
Araneoidea |
| Family |
Nephilidae (Simon, 1894) |
| Subfamily |
Nephilinae |
| Genus |
Nephila (Leach, 1815) |
| Species |
N. pilipes (Fabricius, 1793) |
Synonyms
Due to the many colour variants of N. pilipes (See Section 5), many are commonly thought to be subspecies of N. pilipes but studies have shown that they are identical in almost all aspects, including the epigyne. Thus, many of them are now considered synonyms (See Harvey et al., 2007).[5]
- Aranea longipes (Fabricius, 1781: 545–546) junior primary homonym of Aranea longipes (Fuesslin, 1775)
- Aranea maculata (Fabricius, 1793: 425) junior primary homonym of Aranea maculata (Olivier, 1789)
- Aranea pilipes (Fabricius, 1793: 425)
- Epeira chrysogaster (Walckenaer, 1805: 53–54)
- Epeira sebae (Walckenaer, 1805: 55)
- Nephila fuscipes (Koch, C. L. 1839: 136–138)
- Epeira caliginosa (Walckenaer, 1842: 100)
- Epeira doreyana (Walckenaer, 1842: 100)
- Nephila ornata (Adams, 1847: 291)
- Epeira (Nephila) walckenaeri (Doleschall, 1857: 412)
- Epeira (Nephila) penicillum (Doleschall, 1857: 413)
- Epeira (Nephila) hasseltii (Doleschall, 1859: 27–28)
- Epeira (Nephila) harpyia (Doleschall, 1859: 28)
- Meta ornata (Koch, L. 1872: 134)
- Nephila pecuniosa (Koch, L. 1872: 157–159)
- Nephila aurosa (Koch, L. 1872: 160–162)
- Nephila procera (Koch, L. 1872: 162–163)
- Nephila sulphurosa (Koch, L. 1872: 163–165)
- Nephila tenuipes (Koch, L. 1872: 165–166)
- Nephila maculata annulipes (Thorell, 1881: 146)
- Nephila maculata jalorensis (Simon, 1901: 58)
- Nephila maculata novae-guineae (Strand, 1906: 261–262)
- Nephila pictithorax (Kulczyński, 1911: 469–470)
- Nephila maculata flavornata (Merian, 1911: 195–196)
- Nephila maculata piscatorum (de Vis, 1911: 167–168)
- Nephila maculata lauterbachi (Dahl, 1912: 53)
Aranea pilipes (Fabricius, 1973) is the oldest name without a homonym and hence, will take precedence over other names. In 2005, the species was renamed Nephila pilipes to reflect the new rank Nephilidae.[47]
Subspecies
At present, the only recognised subspecies is Nephila pilipes malagassa (Strand, E., 1907: 533).[48][49] Harvey et al. (2007) did not examine the holotype which was located at Staatliches Museum für Naturkunde, Stuttgart, Germany and thus, the validity of the subspecies status could not be confirmed.[5]
 |
Translation in English (via Google Translate): The mound of Epigyne end up with two narrow, sharp, short, parallel longitudinal impressions (which can sometimes be indicated at f. pr.) The leading edge of the transverse groove of the posterior slope of the mound in the middle higher, sharper, hence, on the edge appearing downward convexly curved plate. Arranged on the front slope of the mound in two transverse row among themselves by more than their diameter distant are small round pits. Abdomen blackish with whitish, wavy or zigzag-shaped, sometimes anastomosing longitudinal strokes. Abdomen not spotted shortly before the spinnerets. - 43mm. Legs: I. 98; II. 81.3; III. at least 47.5mm (incomplete); IV. (?). Madasgascar. |
| Figure 32. Original description of N. pilipes malagassa (Strand, 1907) |
Vernacular (Common) Names
7.3 Etymology
| The genus name Nephila has roots to Ancient Greek where it means "fond of spinning". This is derived from the words νήμα (nema) "thread" + φίλος (philos) "love".[51] N. pilipes is also commonly known as the golden orb/web spider as it spins a circular (orb) web with yellowish-golden coloured silk (Fig. 33).[1] It is also called the giant wood spider because of its large size and also its preference for a woody habitat. |
|
| Figure 33. Golden orb-web spun by N. pilipes (Copyright © 2013 Hewett, N.) |
7.4 Type Specimen
Type specimen Aranea longipes was first described by Fabricius in 1781.[5] The specimen originated from Australia.[52] "Muſ. Dom. Banks." makes a reference to Sir Joseph Banks (1743 - 1820) and that the type specimen Fabricius described came from his collection. Although not confirmed (due to lack of access to Zimsen, 1964), the type locality is likely to be in the British Museum of Natural History, where majority of Banks' collection is kept.[53][54][55]
 |
Translation in English (via Google Translate): A. black, brown, cylindrical abdomen, imprinted with six dots, very long legs. Dwells in Australasia. Body: Large, black. Mandibles: Toothed at the tips, clawed. Pedipalps: Rust brown base, black tips. Abdomen: Cylindrical abdomen with three pairs of brown dots printed on a black dorsal. Feet: Front legs especially elongated, black. |
| Figure 33. Original description of Aranea longipes (Fabricius, 1781) |
8. Useful Links
Encyclopedia of Life (Nephila pilipes)
Wikispecies (Nephila pilipes)
A Guide to Common Singapore Spiders
International Society of Arachnology
9. Literature and References
1. Tan, R. (2001). Mangrove and wetland wildlife at Sungei Buloh Wetlands Reserve. Golden Orb Web Spider. Available from: http://www.naturia.per.sg/buloh/inverts/nephila.htm
2. Kunter, M. & Coddington, J. A. (2009). Discovery of the Largest Orb weaving Spider Species: The Evolution of Gigantism in Nephila. PLoS One. 2009; 4(10): e7516. doi: 10.1371/journal.pone.0007516
3. Robinson, M. H. & Robinson, B. C. (1973). Ecology and behavior of the giant wood spider Nephila maculata (Fabricius) in New Guinea. Smithsonian Contributions to Zoology, 149:1-76.
4. Koh, J. K. H. (2000). A guide to common Singapore spiders. Golden Web Spider. Singapore Science Centre, Singapore. Available from: http://habitatnews.nus.edu.sg/guidebooks/spiders/text/Nephila_maculata.htm
5. Harvey, M. S., Austin, A. D. & Adams, M. (2007). The systematics and biology of the spider genus Nephila (Araneae: Nephilidae) in the Australasian region. Invertebrate Systematics, 21:407-451.
6. Barrion, A. T. & Litsinger, J. A. (1995). Family Araneidae Dahl. In, Riceland Spiders of South and Southeast Asia (pp. 560 - 564). Cambridge, CB: University Press.
7. Chew, T., Chew, S. & Chew, P. (2004). Brisbane Insects and Spiders Homepage. Garden orb web spider - Eriophora transmarina. Available from: http://www.oocities.org/brisbane_weavers/Garden_sp.htm
8. Nieuwenhuys, E. (2011). The Spider. The Web. Available from: http://ednieuw.home.xs4all.nl/Spiders/InfoNed/webthread.html
9. MathScience Innovation Center. (n.d.). Build an Orb Web. Spider Room. Available from: http://www.spiderroom.info/buildanorbweb.html#
10. Zschokke, S. (2000). Form and function of the orb-web. European Arachnology (Toft S & Scharff N, eds). Aarhus University Press, Aarhus: 99-106
11. McCook, H. C. (1889). Procuring food and feeding. In, American spiders and their spinningwork: In, A natural history of the orbweaving spiders of the United States, with special regard to their industry and habits (pg. 249). Philadelphia, PA: McCook, H. C.
12. Robinson, M. H. & Olazarri, J. (1971). Units of Behavior and Complex Sequences in the Predatory Behavior of Argiope argentata (Fabricius): (Araneae: Araneidae). Smithsonian Contributions to Zoology., 65:1-36.
13. Iyengar, E. V. (2008). Kleptoparasitic interactions throughout the animal kingdom and a re-evaluation, based on participant mobility, of the conditions promoting the evolution of kleptoparasitism. Biological Journal of the Linnean Society, 93:745–762.
DOI: 10.1111/j.1095-8312.2008.00954.x
14. Whitehouse, M. (2011). Kleptoparasitic spiders of the subfamily Argyrodinae: a special case of behavioural plasticity. In Herberstein, M. E. (Ed.), Spider Behaviour: Flexibility and Versatility (pg. 363). New York, NY. Cambridge University Press.
15. Hénaut, Y. (2000). Host selection by a kleptoparasitic spider. Journal of Natural History, 34:747–753. DOI: 10.1080/002229300299390
16. Koh, T. H. & Li, D. (2002). Population characteristics of a kleptoparasitic spider Argyrodes flavescens (Araenae: Theridiidae) and its impact on a host spider Nephila pilipes (Araneae: Tetragnathidae) from Singapore. The Raffles Bulletin of Zoology, 50 (1):153-160.
17. Vollrath, F. (1979). Behavior of the kleptoparasitic spider Argyrodes elevatus (Araneae, Theridiidae). Animal Behaviour 27:515–521.
18. Elgar, M.A. (1989). Kleptoparasitism: a cost of aggregating for an orb-weaving spider. Animal Behaviour 37:1052–1055.
19. Whitehouse, M., Agnarsson, I., Miyashita, T., Smith, D., Cangialosi, K., Masumoto, T., Li, D. & Henaut, Y. (2002). Argyrodes: Phylogeny, Sociality and Interspecific Interactions—A Report on the Argyrodes Symposium, Badplaas 2001. The Journal of Arachnology, 30:238-245.
20. Charezieux, B. (1961). Contribution a l'étude du comportement sexuel de l'Argiopide Nephila madagascariensis observé dans son pays d'origine. Bulletin de la Société Zoologique de France, 86:372-379
21. Bristowe, W. S. (1941). The Comity of Spiders. Volume 2. London, LDN. Ray Society.
22. Higgins, L. (2002). Female Gigantism in a New Guinea Population of the Spider Nephila maculata. Oikos, 99:377-385.
23. Robinson, M. H. & Robinson, B. C. (1976). The Ecology and behavior of Nephila maculata: A Supplement. Smithsonian Contributions to Zoology, 218:1-22.
24. Hormiga, G., Scharff, N. & Coddington, J. A. (2000). The phylogenetic basis of sexual size dimorphism in orb-weaving spiders (Araneae, Orbiculariae). Syst. Biol., 49:435-462.
25. Bellis, M. (n.d.). What influenced the Wright Brothers to study flight?. about.com. Available from: http://inventors.about.com/od/weirdmuseums/ig/Wright-Brothers/Influenced-the-Wright-Brothers.htm
26. Pearce, M. (2013). Eastgate Development Harare. Mick Pearce. Available from: http://www.mickpearce.com/works/office-public-buildings/eastgate-development-harare/
27. Richter, J. P. (1888). XIV Anatomy, Zoology and Physiology. In, The Notebooks of Leonardo da Vinci (Volume 2) (pg. 837). Project Gutenberg 2004. Available from: http://www.gutenberg.org/cache/epub/5000/pg5000.txt
28. Shao, Z. & Fritz Vollrath, F. (2002). Materials: Surprising strength of silkworm silk. Nature, 418:741. doi: 10.1038/418741a
29. Porter, D., Vollrath, F. & Shao, Z. (2005). Predicting the mechanical properties of spider silk as a model nanostructured polymer. The European Physical Journal E, 16 (2):199-206.
30. Yang, Y., Chen, X., Shao, Z., Zhou, P., Porter, D., Knight, D. P. & Vollrath, F. (2005). Toughness of Spider Silk at High and Low Temperatures. Adv. Mater., 17:84–88. doi: 10.1002/adma.200400344
31. Emile, O., Le Floch, A. & Vollrath, F. (2005). Biopolymers: Shape memory in spider draglines. Nature, 440:621. doi:10.1038/440621a
32. Allmeling, C., Jokuszies, A., Reimers, K., Kall, S. & Vogt, P. M. (2006). Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. Journal of Cellular and Molecular Medicine, 10:770–777. doi: 10.1111/j.1582-4934.2006.tb00436.x
33. Hansel, B. (2013). Kraig Biocraft Laboratories Launches Commerical Production of Monster Silk™. Kraig Biocraft Laboratories. Available from: http://www.kraiglabs.com/kraig-biocraft-laboratories-launches-commercial-production-of-monster-silktm/
34. Lewis, R. (n.d.). Department of Molecular Biology. University of Wyoming. http://www.uwyo.edu/molecbio/faculty-and-staff/randy-lewis.html
35. Zyga, L. (2010). Scientists breed goats that produce spider silk. Phys.org. http://phys.org/news194539934.html
36. Xia, X., Qian, Z., Chang, S. K., Young, H. P., Kaplan, D. L., & Sang, Y. L. (2010). Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. PNAS, 107 (32):14059-14063. doi: 10.1073/pnas.1003366107
37. Starmer, C. F. (n.d.). Nephila anatomy. MUSC IT Lab. Available from: http://frank.itlab.us/learning/nephila_anatomy.pdf
38. Foelix, R. F. (2010). Functional Anatomy. In, Biology of Spiders (3rd Edition) (pp. 17 - 48). New York, NY. Oxford University Press.
39. Comstock, J. H. (1912). The spider book. New York, NY: Doubleday, Page & Company. DOI: http://dx.doi.org/10.5962/bhl.title.3163
40. Koh, J. K. H. (2000). A guide to common Singapore spiders. Batik Golden Web Spider. Singapore Science Centre, Singapore. Available from: http://habitatnews.nus.edu.sg/guidebooks/spiders/text/Nephila_antipodiana.htm
41. Koh, J. K. H. (2000). A guide to common Singapore spiders. Golden Web Spider. Singapore Science Centre, Singapore. Available from: http://habitatnews.nus.edu.sg/guidebooks/spiders/text/Nephila_maculata.htm
42. Levi, H. W., & von Eickstedt, V. R. D. (1989). The Nephilinae spiders of the neotropics (Araneae: Tetragnathidae). Memorias do Instituto Butantan, 51:43–56.
43. Hormiga, G., Eberhard, W. G. & Coddington, J. A. (1995). Web construction Behaviour in Australian Phonognatha and the Phylogeny of Nephiline and Tetragnathid Spiders (Araneae: Tetragnathidae). Australian Journal of Zoology, 43:313-364. doi: 10.1071/ZO9950313
44. Pan, H., Zhou, K. Y., Song, D. & Yang, Q. (2004). Phylogenetic placement of the spider genus Nephila (Araneae: Araneoidea) inferred from rRNA and MaSp1 gene sequences. Zoological Science, 21:343-351. doi: 10.2108/zsj.21.343
45. Kuntner, M., Coddington, J. A. & Hormiga, G. (2008). Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics, 24:147-217. doi: 10.1111/j.1096-0031.2007.00176.x
46. Su, Y. C., Chang, Y. H., Smith, D., Zhu, M. S., Kuntner, M. & Tso, I. M. (2011). Biogeography and speciation patterns of the golden orb spider genus Nephila (Araneae: Nephilidae) in Asia. Zoological Science, 28:47-55.
47. Kuntner, M. (2005). Systematics and evolution of nephilid spiders (Araneae, Nephilidae new rank). Washington DC: George Washington University.
48. Integrated Taxonomic Information System. (n.d.). Nephila pilipes malagassa. Available from: http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=871238#
49. Strand, E. (1907). Vorläufige Diagnosen afrikanischer und südamerikanischer Spinnen. Zoologischer Anzeiger, 31:525–558.
50. Enyclopedia of Life. (n.d.). Nephila pilipes. Names. Available from: http://eol.org/pages/1193401/names
51. Cameron, H. D. (2005). An etymological dictionary of North American spider genus names. In Ubick, D., Paquin, P., Cushing, P. E. et al. (Eds.), Spiders of North America: An identification manual. American Arachnological Society.
52. Fabricius, J. C. (1781). Species insectorum exhibentes eorum differentias specificas, synonyma auctorum, loca natalia, metamorphosin adiectis observationibus, descriptionibus Tom. I. Hamburgi et Kilonii, Carol. Ernest. Bohnii.
53. Zimsen, E., (1964). The type material of J. C. Fabricius. Copenhagen: Munksgaard.
54. Tuxen, S. L., (1967). The Entomologist, J. C. Fabricius. Annual Review of Entomology, 12:1-15. doi: 10.1146/annurev.en.12.010167.000245
55. O'Shea, R. (1973). Type designations of some Fabrician Coreidae in the Banks Collection at the British Museum (National History). Annals of the Entomological Society of America, 66(5):1177-1178(2)
56. Kuntner, M., Shichang, Z., Gregorič, M. & Li, D. (2012). Nephila female gigantism attained through post-maturity molting. Journal of Arachnology, 40 (3):345-347
9.1 Copyrights of Images and Illustrations
1. Starmer, F. (2008). Permission is granted to copy, distribute and/or modify this document under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
2. Hamm, N. (2002). Permission to publish image sought but not granted because author is uncontactable. However, usage of the image is within the terms of fair use. http://www.spiderroom.info/buildanorbweb.html#
3. Rentz, D. (2013). Permission is granted by author on 24 Nov 2013 to copy, distribute and/or modify this document under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
4. Hewett, N. (2013). Permission to publish image sought and granted by author on 17 Nov 2013. Copyright remains with Mr Neil Hewett. http://www.ccwild.com/spiders/
5. Pearson, S. & Pearson, A. (2013). Permission to publish image sought and granted by authors on 19 Nov 2013. Copyright remains with the authors, Steve Pearson and Alison Pearson, Airlie Beach, Australia.
6. Whyte, R. (2013). Permission to publish image sought and granted by author on 18 Nov 2013. Copyright remains with Mr Robert Whyte. http://www.arachne.org.au/01_cms/details.asp?ID=1627#11619
7. Benjamint444. (2010). Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, version 1.2 only.
Author
Name: Wilson Wee
Email: wilson.weews@gmail.com







